The FDA officially unveiled its proposal requiring front-of-package nutrition labels, the agency said in a news release on Tuesday, Jan. 14. This effort is part of a broader strategy to combat chronic diseases, which affect about 60 percent of Americans and account for $4.5 trillion in annual health care costs.
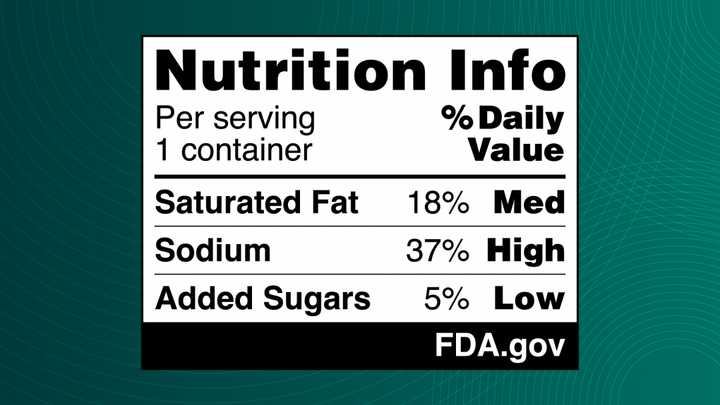
The proposed "nutrition info box" would display saturated fat, sodium, and added sugars content in a clear, black-and-white format, categorizing levels as low, medium, or high.
"The science on saturated fat, sodium, and added sugars is clear," said FDA Commissioner Dr. Robert Califf. "Nearly everyone knows or cares for someone with a chronic disease that is due, in part, to the food we eat. It is time we make it easier for consumers to glance, grab and go. Adding front-of-package nutrition labeling to most packaged foods would do that."
The simplified approach complements the more detailed nutrition facts label, providing consumers with a quick way to assess the healthfulness of products. Research has consistently linked excessive consumption of saturated fat, sodium, and added sugars to chronic diseases like heart disease, cancer, and diabetes.
A 2023 FDA study involving nearly 10,000 adults revealed that the proposed label design helped consumers identify healthier options more accurately and efficiently than other formats tested.
"Food should be a vehicle for wellness, not a contributor of chronic disease," said Jim Jones, the FDA's deputy commissioner for human foods. "In addition to our goal of providing information to consumers, it’s possible we’ll see manufacturers reformulate products to be healthier in response to front-of-package nutrition labeling. Together, we hope the FDA’s efforts, alongside those of our federal partners, will start stemming the tide of the chronic disease crisis in our country."
Australia, Chile, Ecuador, France, and the United Kingdom are among dozens of countries that already have similar front-of-package label rules, according to the Center for Science in the Public Interest. The labeling has been credited with reducing the daily rate of purchased sodium, saturated fat, and calories from sugar in packaged foods.
The proposal aligns with the White House National Strategy on Hunger, Nutrition, and Health, which aims to reduce diet-related diseases by 2030. It complements other FDA initiatives, including updated "healthy" claims and voluntary sodium reduction targets, all aimed at empowering consumers and improving the food supply.
If finalized, manufacturers would have three years to comply if their annual food sales exceed $10 million. Food producers with sales less than that would have four years to adapt to the rule.
Public comments on the proposal are open until Friday, May 16, and can be made at regulations.gov.
Click here to follow Daily Voice Watertown and receive free news updates.